|
Surprisingly, we are back already to talk about dexamethasone. Our last podcast, #204, covered the press release from the RECOVERY (Randomised Evaluation of COVid-19 thERapY) trial and their mentioning of results regarding dexamethasone. Now, the study is available in a pre-print form. It is important that this has not yet been peer-reviewed and is still preliminary, but we now have some real information that we can review!
Title:
Horby, et al. Effect of Dexamethasone in Hospitalized Patients with COVID-19 – Preliminary Report Case: You are treating a patient with COVID-19 that has been intubated, sedated, and on invasive mechanical ventilation (IMV) but continues to worsen. A recent paper has been discussed in the news about an old and established corticosteroid that may help, dexamethasone. You question if this may help save your patient's life and if this should be used for this patient. Background: The COVID-19 pandemic has been devastating and treatment has been controversial. Until this time, no medications had shown improvement in survival. Dexamethasone has been discussed in previous podcasts including #24 covering single dose dexamethasone in adult asthma, #53 on oral dexamethasone for sore throats, and #121 when discussing the management of croup. COVID-19 has also been discussed before on podcasts #189, #190, #192, #193, #194, #195, #196, #197, #199, and #200 in addition to our last podcast. Clinical Question: Does dexamethasone (IV or PO) + usual care reduce 28 day mortality in patients hospitalized with COVID-19 compared to usual care alone? Reference:
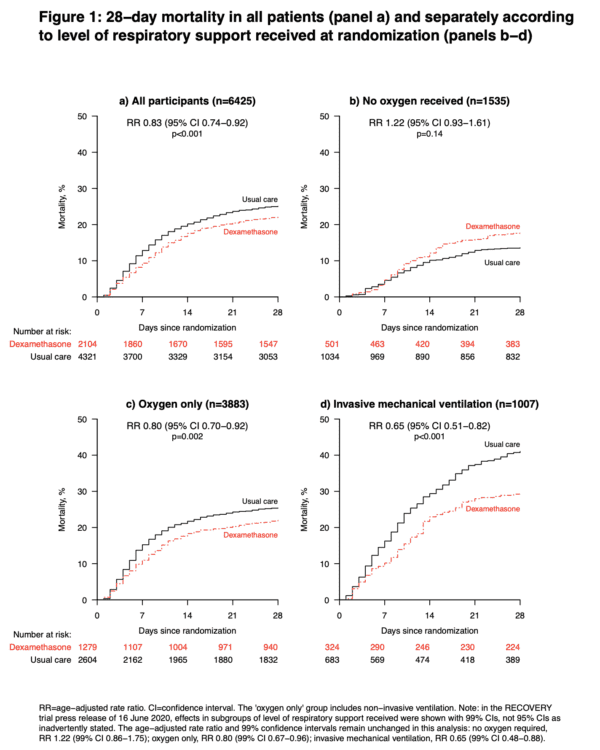
Author’s Conclusions: “In patients hospitalized with COVID-19, dexamethasone reduced 28-day mortality among those receiving invasive mechanical ventilation or oxygen at randomization but not among patients not receiving respiratory support.” Quality Checklist for Randomized Clinical Trials:
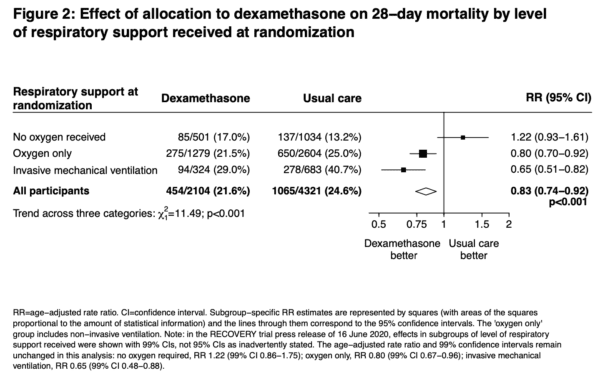
Key Results: 2104 patients randomly allocated to receive dexamethasone were compared with 4321 patients concurrently allocated to usual care. Overall, 454 (21.6%) patients allocated dexamethasone and 1065 (24.6%) patients allocated usual care died within 28 days (age adjusted rate ratio [RR] 0.83; 95% confidence interval [CI] 0.74 to 0.92; P<0.001, fragility index [FI] 18, number needed to treat [NNT] of 33.3). The proportional and absolute mortality rate reductions varied significantly depending on level of respiratory support at randomization (test for trend p<0.001): Dexamethasone reduced deaths by one-third in patients receiving invasive mechanical ventilation (29.0% vs. 40.7%, RR 0.65 [95% CI 0.51 to 0.82]; p<0.001, FI 17, NNT 8.5), by one-fifth in patients receiving oxygen without invasive mechanical ventilation (21.5% vs. 25.0%, RR 0.80 [95% CI 0.70 to 0.92]; p=0.002, FI 8, NNT 28.6), but did not reduce mortality in patients not receiving respiratory support at randomization (17.0% vs. 13.2%, RR 1.22 [95% CI 0.93 to 1.61]; p=0.14). No FI or NNT calculated with the last group since it was not statistically significant based on the p-value.
Key Points of Debate:
Comparing Conclusions:
We agree that in hospitalized patients with COVID-19, dexamethasone reduces 28-day mortality especially in those receiving IMV and to a lesser degree those receiving oxygen alone. It does not appear to help those not receiving respiratory support. Our Bottom Line: This was a well done RCT for patients with COVID-19 pneumonia. It appears, based on the study that has not yet been peer reviewed, that dexamethasone does significantly improve 28-day mortality for those requiring respiratory support. This is especially true if they are requiring IMV. Case Resolution: You have a discussion with the family by phone to discuss options for management. With shared decision making they elect to start dexamethasone treatment. Thanks to great critical care (and potentially the dexamethasone) your patient is able to successfully recover and survive their ordeal with COVID-19. Clinical Application: While this is a preliminary report it appears that patients requiring any oxygen therapy, HFNC, NIV, IMV, or ECMO would benefit from dexamethasone. However, we must be mindful that further evidence could later disprove this benefit and we should continue to monitor the literature. What do I tell my patient? With your patient being intubated and sedated, there is not much you can tell them. However, you can speak with the family. You explain to the family that there is a steroid named dexamethasone that has been studied and based on the current evidence it has demonstrated that it can save lives in those requiring respiratory support. It is important to emphasize that the evidence may change with time and that there are other factors that can play a role in saving someone's life with COVID-19 pneumonia. Conclusion: This study has been incredibly popular and several posts have already been made including by REBEL EM, FOAMcast, and Broome Docs. Feel free to check these out and make sure to read the article yourself! Let us know what you think by giving us feedback here in the comments section or contacting us on Twitter or Facebook. Remember to look us up on Libsyn and on Apple Podcasts. If you have any questions you can also comment below, email at [email protected], or send a message from the page. We hope to talk to everyone again soon. Until then, continue to provide total care everywhere.
1 Comment
|
Libsyn and iTunesWe are now on Libsyn and iTunes for your listening pleasure! Archives
August 2022
Categories |
||||||


 RSS Feed
RSS Feed